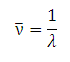Ultraviolet (UV) radiation is ofhigher energy than visible radiation. Both are higher in energy than IR radiation. Ultraviolet radiation has frequencies between, approximately, 7.5 × 10
14 - 1.5 × 10
15 Hz corresponding to wavelengths of 400 nm and 200 nm. Visible radiation has frequencies between approximately 3.7 × 10
14 and 7.5 × 10
14 Hz corresponding to wavelengths of 800 nm and 800 nm. Note that a short wavelength corresponds to a high frequency (and energy) and a long wavelength corresponds to a low frequency (and energy).
Spectroscopists tend to use wavelength (in nm) or "wavenumbers". A wavenumber is the inverse of the wavelength,
λ, in cm:
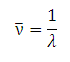
|
|
It has units of 1/cm or cm-1. It is directly proportional to the frequency and the energy of the radiation: radiation with a high wavenumber has higher frequency and energy than radiation with a low wavenumber. Ultra-violet radiation has wavenumbers between, approximately, 25000 - 50000 cm-1. Visible radiation has wavenumbers between, approximately, 12500 - 25000 cm-1.
|
In a typical UV / visible spectrometer, the sample is exposed to radiation in these ranges. When the energy matches that required to excite an electron from one level to another, absorption occurs. Otherwise, the radiation passes through and no absorption occurs. A typical spectrum is shown below. The wavelength or wavenumber of the radiation is plotted on the x-axis. The amount of radiation absorbed at each wavelength or wavenumber is plotted on the y-axis. At wavelength or wavenumbers where no absorprion occurs, the absorption is zero. An absorption corresponds to a 'peak' in the curve.
Most UV / visible spectra are similar to the one shown opposite, consisting of one or more fairly broad bands. The strength of the bands is a characteristic property of a molecule and is used in analytical applications to quantitatively determine concentration.
The use of UV / visible spectroscopy in organic and biochemistry is fairly straightforward:
The presence of strong bands in the UV / visible spectrum of a compound indicates the presence of conjugation.
|
|
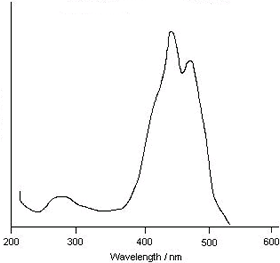
UV / visible spectrum of β-carotene |